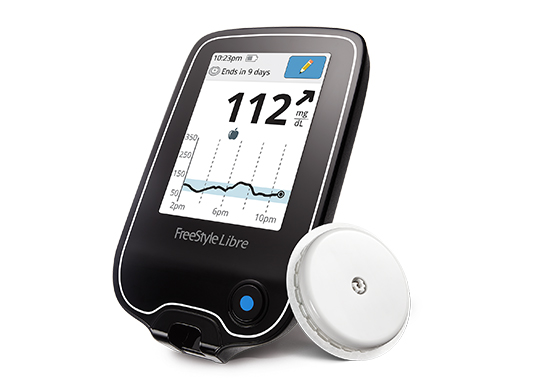
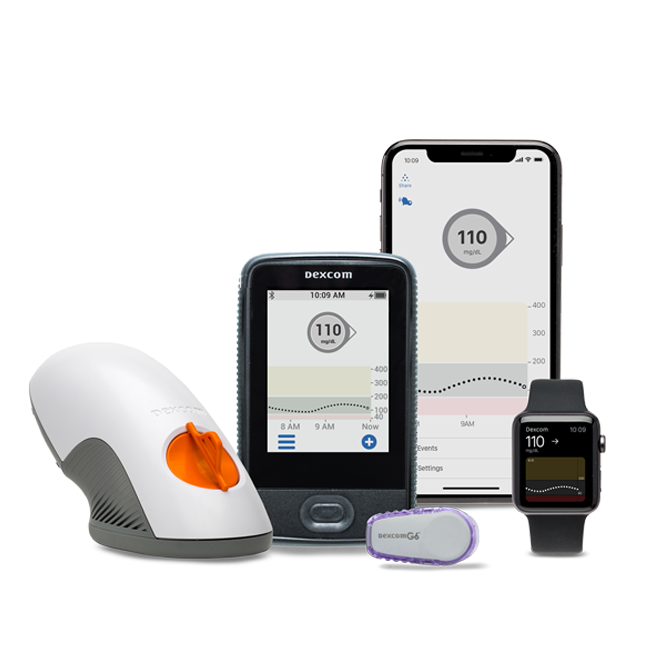
Everyone with diabetes on insulin therapy (or a medication that can cause low blood sugar) should know the signs, symptoms and treatments for hypoglycemia. New glucagon options to treat severe cases are already on the market, and more are on the way.
read more →